

Debates about genetic engineering often invoke the concept of the “perfect child” — the prospect that parents will design future generations to accord with their ideals of appearance, intellect, or health. Yet what most every parent already considers “perfect” is his or her own child. Indeed, the creators of reproductive technology have already gone to great lengths to provide parents with their “own” genetic children. It can be easy to forget that it is this aim, rather than creating designer babies, that still dominates assisted reproductive technology today.
One recent innovation that illustrates the fertility industry’s efforts to establish genetic parenthood is a set of in vitro fertilization procedures that can create children with not two but three genetic parents. Scientists argue that these procedures, which they usually call mitochondrial replacement therapy, could help prevent certain kinds of genetic disease caused by mutations in a woman’s mitochondrial DNA.
The debate over so-called “three-parent babies” has largely been about whether these procedures constitute a defensible therapy or rather a heritable modification of the human germline — a radical transformation of human nature. For example, Shoukhrat Mitalipov, of Oregon Health and Science University, seems concerned mainly with warding off the specter of designer babies when he says of the procedure, “We don’t think this is modification. It’s not something that’s synthetic. It’s just taking a donor genome from somebody and replacing it with one that already exists. It’s natural.”
The trouble is that the conventional therapy-versus-modification framing, broadly accepted by critics and defenders alike, is miscast. As we will see, mitochondrial replacement therapy is not a true therapy, for it is not primarily aimed at preventing disease. But for the same reason, we can see that it is also not the kind of genetic engineering that could lead to designer babies. Instead, mitochondrial replacement is an extension of the many reproductive technologies that have been developed to help individuals and couples have children with specific kinds of biological and social relationships. We might call these technologies not genetic engineering but kinship engineering.
To understand mitochondrial replacement therapy, also known as three-parent IVF, we need a brief gloss on cells and genes. Although the vast majority of genes reside in the chromosomes, there are also a small number in the mitochondria, which are tiny cellular parts that help supply energy to the cell and the body. Scientists estimate that human mitochondrial DNA carries only 37 genes, while the chromosomes have around twenty thousand. But because of the crucial role mitochondria play in metabolism, mutations in their genes can cause serious diseases, such as Leigh syndrome, a neurological disorder that kills most affected children by the age of two or three.
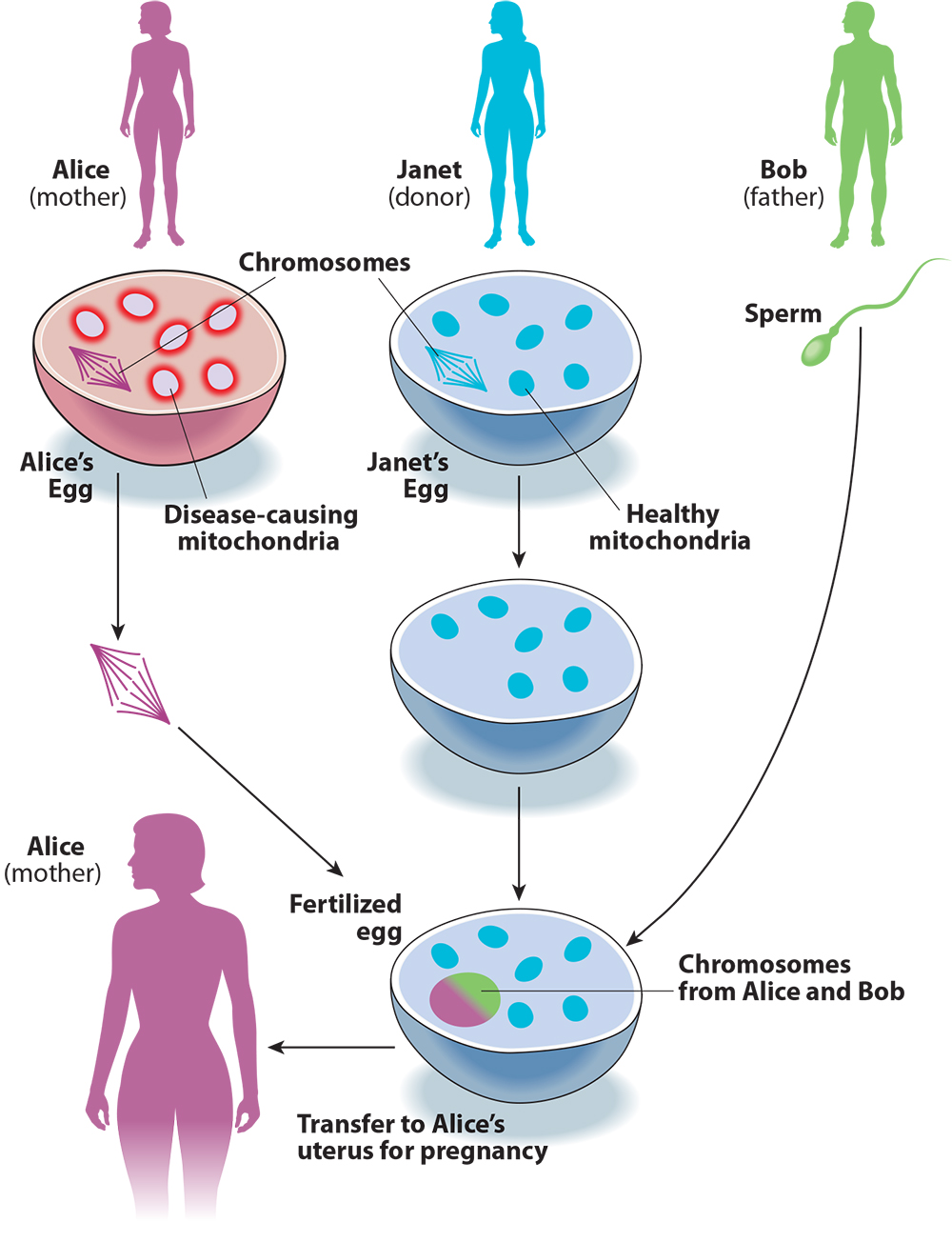
Keeping track of the DNA-swapping in three-parent IVF can be confusing, so let’s try to make sense of it with an example (see Figure 1). A couple, Alice and Bob, want to have a child. Because of a family history of Leigh syndrome, they use genetic testing and find that mutations in Alice’s mitochondria also make her a carrier for the disorder. To avoid passing this disease on to their child, Alice and Bob decide to use three-parent IVF.
They find an egg donor, Janet. Doctors collect eggs from Alice and Janet, then remove the chromosomes from both eggs. Then Alice’s chromosomes are moved into Janet’s egg cell. The reconstructed egg cell is then fertilized with Bob’s sperm. Finally, the resulting embryo is transferred to Alice’s uterus. The embryo will have a large number of genes from Bob and Alice, but also a small number from Janet, via her mitochondria.
Figure 1. Three-Parent IVF
It can be difficult to predict when a child will be affected by mitochondrial diseases, since they have a different inheritance pattern than other genetic conditions. Diseases caused by mutations in mitochondria are passed on only from the mother. But each egg cell contains hundreds of thousands of mitochondria, each of which may or may not contain a disease-causing mutation. This is different from other genes, of which each person carries only two copies, meaning that the relatively straightforward Mendelian logic of recessive and dominant genes determines whether a child will be affected. By contrast, whether a child will be affected by mutations in mitochondria is determined by the proportion and distribution of mitochondria in the egg cell that contain disease-causing genes. Although women who have only a small amount of mutated mitochondria may themselves be healthy, relatively large amounts of mutated mitochondria can end up in some of their egg cells, causing their children to suffer from mitochondrial disease.
Returning to Alice, Bob, and Janet, if Alice has reason to suspect that she is at risk of passing mitochondrial disease to her children, she can use these three-parent IVF techniques to make sure that her kids have her own chromosomes. But instead of Alice’s mutated, disease-causing mitochondria, they will have Janet’s healthy mitochondria. Compared to natural conception between Alice and Bob, this procedure reduces the chance that the resulting child will have a mitochondrial disease inherited from Alice.
However, it doesn’t necessarily eliminate that chance. In a 2016 paper, scientists provided evidence that some mutated mitochondria can be carried along with the chromosomes and that even small amounts might cause disease. And it remains unclear what risks of birth defects and developmental problems could be posed by all the manipulations of egg cells and embryos involved in three-parent IVF.
In 2015, the United Kingdom became the first country to legalize mitochondrial replacement therapies. In the United States, before they could be used, Congress banned the Food and Drug Administration from approving any kinds of reproductive techniques “in which a human embryo is intentionally created or modified to include a heritable genetic modification.” Earlier this year a Democrat-controlled House spending panel tried to repeal the ban — which a representative of the American Society for Reproductive Medicine, an industry advocacy group, described as “an antiscience rider” — but their efforts ultimately failed, and so these procedures remain illegal in the United States, for now. The first three-parent baby was born in April 2016 to a Jordanian couple, through a procedure performed in Mexico by a team of doctors from New York. The mother was a carrier for Leigh syndrome, and two of the parents’ previous children had died from the disease.
Proponents of three-parent IVF argue that it will prevent mitochondrial diseases. This is certainly an effective rhetorical strategy. As an April editorial in the New York Times observes, “Surveys have consistently shown that most Americans support the use of technology that modifies the human genome, as long as it’s to eliminate diseases.” And the diseases that three-parent IVF is meant to prevent can be very serious. A therapy that could protect children from a brutal disease such as Leigh syndrome would be worth pursuing, even if it did pose some risks.
But the term “mitochondrial replacement therapy” is misleading, for two reasons. First, what is being replaced is not in fact the mitochondria but rather the chromosomes: It is Janet’s egg that becomes the embryo, with Alice’s chromosomes inserted in place of Janet’s (see Figure 1 again). Second, the need fulfilled by the procedure is not therapy — in this case eliminating the risk of mitochondrial disease — but creating a genetic relationship between the child and the birth mother, Alice.
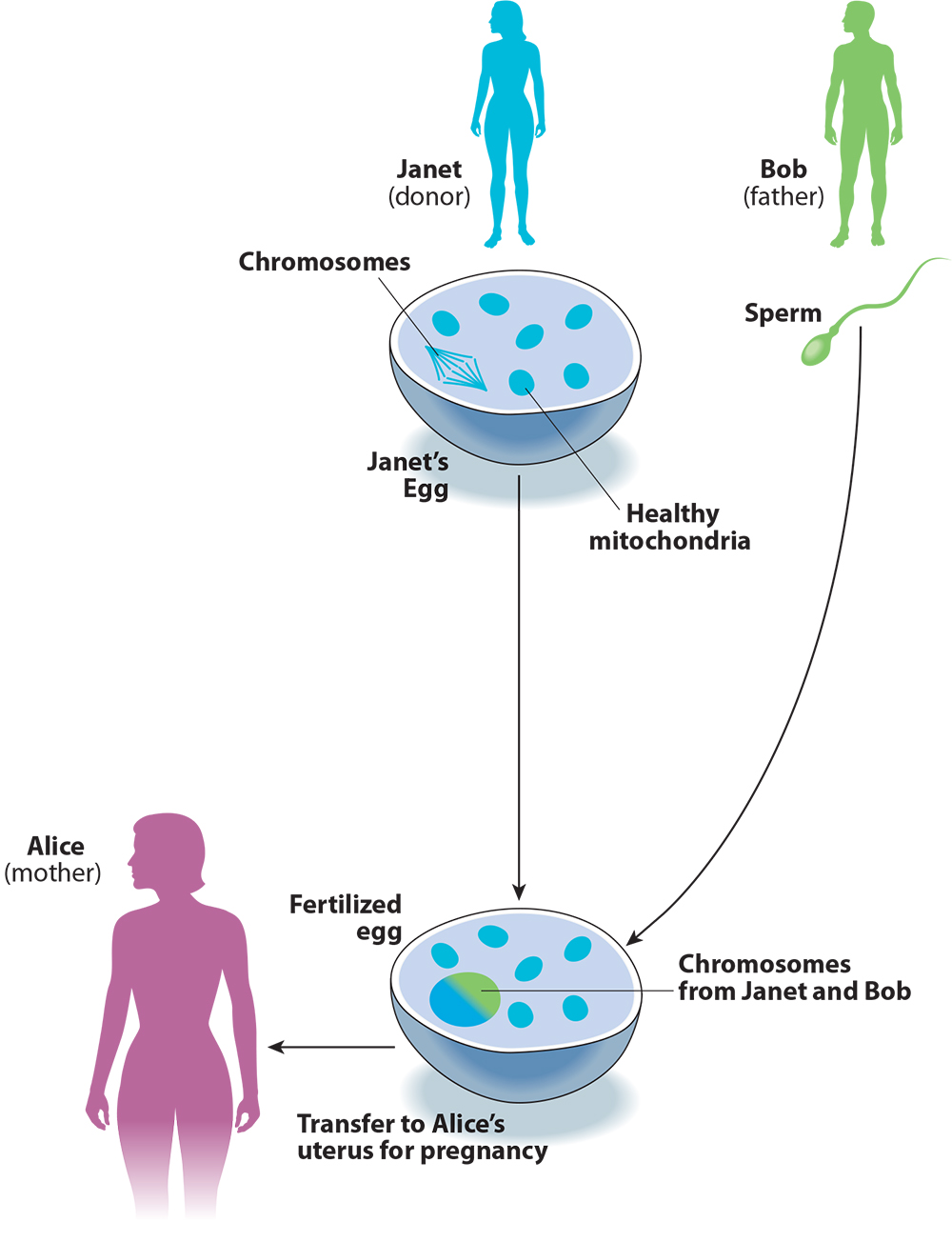
These two points become clear when we compare the procedure to conventional egg donation with IVF (see Figure 2). Imagine if Alice and Bob took the donated egg from Janet, fertilized it with Bob’s sperm, and transferred it to Alice’s uterus, without any of the chromosome-swapping involved in three-parent IVF. This is how conventional IVF works for the thousands of infertile couples who use donor eggs in America every year. For Alice and Bob, this procedure would not just reduce but entirely eliminate the risk that the child would inherit mitochondrial disease from Alice, and would not pose any risks beyond those of IVF itself. The advantage for Alice and Bob of using three-parent instead of conventional IVF is that the child will be genetically related to Alice. Calling this process “mitochondrial replacement therapy” helps to present the procedure as a way of preventing mitochondrial disease, when in fact it is a method for engineering the child’s genetic relationships.
Figure 2. Conventional IVF with Egg Donation
The distinction matters a great deal for thinking about whether or not the procedure is medically justified. If we believe the purpose of three-parent IVF to be the prevention of disease, then we should compare the risks of the procedure to the dangers of the disease. This is how we think about medicine in most cases: A dangerous treatment like a bone marrow transplant can be justified for cancer, but not for a less serious condition. When we compare the dangers of three-parent IVF to the dangers of mitochondrial disease, it’s easy to see how the procedure could be justified. This was the logic offered by bioethicist Arthur Caplan, for example, when he told CNN that the technique is “not without its risks, but it’s treating a disease.”
But if we consider the real purpose of three-parent IVF to be ensuring that the child has a genetic relationship to the mother, the ethical consideration shifts: How do we compare the dangers of three-parent IVF to the disadvantages of the child not being genetically related to the mother? Or, to put it differently: How do we compare the benefit of the child’s being genetically related to the mother to the risk of even small amounts of mutated mitochondria causing disease in the child? There is no straightforward way to compare the non-medical interest of the parents in having a genetically related child to the medical risks of the three-parent IVF procedure. Dangerous therapies for treating dangerous diseases may make sense. But how do we make sense of dangerous forms of engineering kinship relations?
Like other reproductive technologies, three-parent IVF is meant to provide couples with a healthy, genetically related child. And, like other reproductive technologies, it prioritizes the genetic relationship. If the aim were simply to eliminate the risk of a genetic disease, the couple could ensure this by using donor eggs or sperm, or by adopting. The strangeness of three-parent IVF helps us to see more clearly what the fertility industry has been offering all along.
That the technique is meant more as a new way for the fertility industry to offer kinship engineering than as a novel way of preventing disease can be seen in how it is already being used as a fertility treatment for women who have trouble getting pregnant using their own egg cells. Nuno Costa-Borges, a scientist who recently performed three-parent IVF for infertile patients in clinical trials in Greece, said in an interview that these techniques “may represent a new era in the IVF field, as it could give these patients chances of having a child genetically related to them.” Since both infertile women and women with mitochondrial disease could use regular IVF with unmodified donor eggs to have a child, three-parent IVF offers each the same advantage: to have a genetically related child.
But the child created by three-parent IVF presents us with a form of genetic kinship never before seen in the history of life. In creating this novel relation, the fertility industry extends its promise of kinship to new categories of prospective parents. For example, three-parent IVF easily suggests itself to allowing lesbian couples to have children genetically related to both partners. One of the women would be the mitochondrial donor while the other would provide the chromosomal DNA. The two women would make unequal genetic contributions to their child, but both would contribute, unlike with conventional IVF, in which the child would be related to only one of them.
Bioethicists Giulia Cavaliere and César Palacios-González recently argued in the Journal of Medical Ethics that three-parent IVF is justified not by its putative therapeutic purpose of preventing mitochondrial disease, but by the reproductive freedom of parents to secure the kinds of kinship they desire. Therefore, they argue, three-parent IVF should be offered to lesbian couples who want both partners to be genetically related to their child. Many advocates of three-parent IVF downplay the familial significance of the small number of genes in mitochondria, and might well dismiss the use of such technology by lesbian parents as “a very expensive vanity project,” as Cavaliere and Palacios-González put it. But the authors maintain that this tiny portion — far less than one percent of genes are mitochondrial — “suffices for establishing genetic parenthood.”
Egg donation is itself controversial, and for good reasons. The procedure for collecting egg cells subjects donors to considerable risks, and they take on those risks not for their own health but to advance the reproductive projects of another person. In conventional IVF, the donors also give up their rights and responsibilities to raise their own genetic children, who are themselves deprived of a relationship with their genetic parents. The decision to give up one’s genetic children in egg and sperm donation is not made with the child’s best interests in mind, but rather with the interests of the adults who want to be (or not to be) considered the child’s parents.
Some of these problems with egg donation also apply to three-parent IVF. The procedure makes use of donated eggs, so it exposes egg donors to the same risks involved with collecting them. The egg donor will have less of a genetic relationship with the child than she would have with conventional IVF, but will still have some genetic relationship.
Just what that relationship is can be difficult to think about. On the one hand, there are very few mitochondrial genes, and they do not appear to influence easily observable traits like appearance. Some scientists, writing in a 1998 paper, found evidence that mitochondrial DNA could be associated with intelligence, and it is certainly true that mitochondria — the “powerhouses of the cell,” as they are often called — play an important role in many parts of the body. But with a few exceptions, the differences between the mitochondrial genes of different people don’t make much of a difference to their physiology and behavior. One of the big exceptions is of course mitochondrial disease, where the difference between healthy mitochondria and mitochondria with disease-causing mutations can be a matter of life or death. In those cases where three-parent IVF is used to prevent mitochondrial disease, the child inherits something very important from the egg donor.
A child conceived through three-parent IVF “inherits” also something else: Unlike the DNA found on the chromosomes, mitochondrial DNA does not get recombined with each generation, but is rather passed directly between mothers and daughters through the generations. A child who discovers this fact about herself might wonder about the long maternal lineage she is connected to through her anonymous mitochondrial donor. Even if this idea of unbroken lineages of mitochondrial DNA comes from science rather than ordinary lived experience, we don’t know what it might mean for children born through three-parent IVF.
Three-parent IVF ensures that the mother will have a genetic relationship with her child, which she would not have had if unmodified donor eggs had been used. But is the problem with regular egg donation that the parents will raise a child who is not genetically related to the mother, or is it the alienation of the child from her genetic mother? Another way to put it: Are parents entitled to children with both parents’ genes, or are children entitled to a relationship with their biological parents? Three-parent IVF aims to resolve the first matter, but does not resolve the latter, since there will still be a genetic relationship between the child and the anonymous egg donor.
If we are to understand three-parent IVF as not truly a procedure for preventing genetic disease, we must ask: How far should we go to secure genetic kinship for prospective parents? Is this really a legitimate goal for medicine? And what does this goal tell us about the value we place today on ties to children who aren’t genetically related to their parents? As bioethicist Françoise Baylis asked in an interview last year, “If we continue to emphasize that genetic ties are important, what are we saying to all of the people who currently use assisted reproductive technologies with donor eggs and donor sperm? What are we saying to all the people who currently choose not to use reproductive technologies, but to adopt?”
Baylis’s question points to an irony in how the fertility industry has shifted from justifying practices like egg donation to pushing for three-parent IVF. For decades, thousands of infertile couples have had to rely on donated eggs or sperm so that they can create a child genetically related to at least one parent — even if this means that the child’s other genetic parent is a donor he or she never even meets. The industry and its allies have long sought a delicate balance of sentiments, in which a genetic relationship between parent and child should be celebrated and secured at great expense when current technology can achieve it, but its absence largely shrugged off when technology can’t. Meanwhile, children themselves should not be too concerned about their merely genetic relationship to an often anonymous sperm or egg donor. What matters to the fertility industry are the interests of the parents in having a child who will be genetically related to at least one of them; the interest of children in having a relationship with both — or all three — of their genetic parents is decidedly secondary.
As new technologies offer us more and more power over human genetics and reproduction, questions about whether we will exercise that power over future generations responsibly become more urgent. Will we use technologies like CRISPR to give children the best chances for health and happiness, or will this power be used to manufacture children in accordance with the present generation’s whims and preferences? The willingness of the fertility industry to use experimental technologies like three-parent IVF to satisfy the kinship desire of prospective parents, even when it means putting the health of children at risk, bodes ill for how they will use the even more powerful technologies of genetic engineering now on the horizon.
During Covid, The New Atlantis has offered an independent alternative. In this unsettled moment, we need your help to continue.